
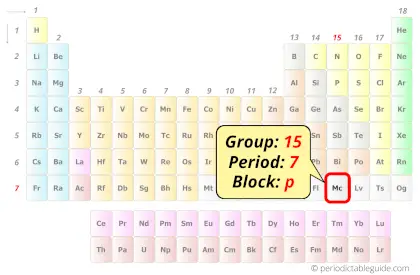
This means that the nucleus attracts the electrons more strongly, pulling the atom's shell closer to the nucleus. The effect of increasing proton number is greater than that of the increasing electron number therefore, there is a greater nuclear attraction. However, at the same time, protons are being added to the nucleus, making it more positively charged. This is because, within a period or family of elements, all electrons are added to the same shell. Atomic radius patterns are observed throughout the periodic table.Ītomic size gradually decreases from left to right across a period of elements. The covalent radii of these molecules are often referred to as atomic radii. Unlike the two previous 7p elements, moscovium is expected to be a good homologue of its lighter congener, in this case bismuth.
#Mc periodic table series#
Nevertheless, it is possible for a vast majority of elements to form covalent molecules in which two like atoms are held together by a single covalent bond. Our New Periodic Table - Recently IUPAC announced the name of four following elements - Nihonium (Nh), for the element 113, Moscovium (Mc), for the. Moscovium is predicted to be the third member of the 7p series of chemical elements and the heaviest member of group 15 in the periodic table, below bismuth. Some are bound by covalent bonds in molecules, some are attracted to each other in ionic crystals, and others are held in metallic crystals. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Moscovium was named after the Moscow region, the. The atomic radius is one-half the distance between the nuclei of two atoms (just like a radius is half the diameter of a circle). The four atoms of element 115 was produced by bombardment of targets of americium-243 by calcium-48 ions. This is caused by the increase in atomic radius. Electron affinity decreases from top to bottom within a group.This is caused by the decrease in atomic radius. Electron affinity increases from left to right within a period.This causes the electron to move closer to the nucleus, thus increasing the electron affinity from left to right across a period. Moving from left to right across a period, atoms become smaller as the forces of attraction become stronger. With a larger distance between the negatively-charged electron and the positively-charged nucleus, the force of attraction is relatively weaker. This means that an added electron is further away from the atom's nucleus compared with its position in the smaller atom. \( \newcommand\): Periodic Table showing Electron Affinity TrendĮlectron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend, discussed below). The Chemistry Divisions Periodic Table describes the history, properties.


 0 kommentar(er)
0 kommentar(er)
